In Group 1 the reactivity of the elements increases going down the group. They react with oxygen to form the primary oxides.
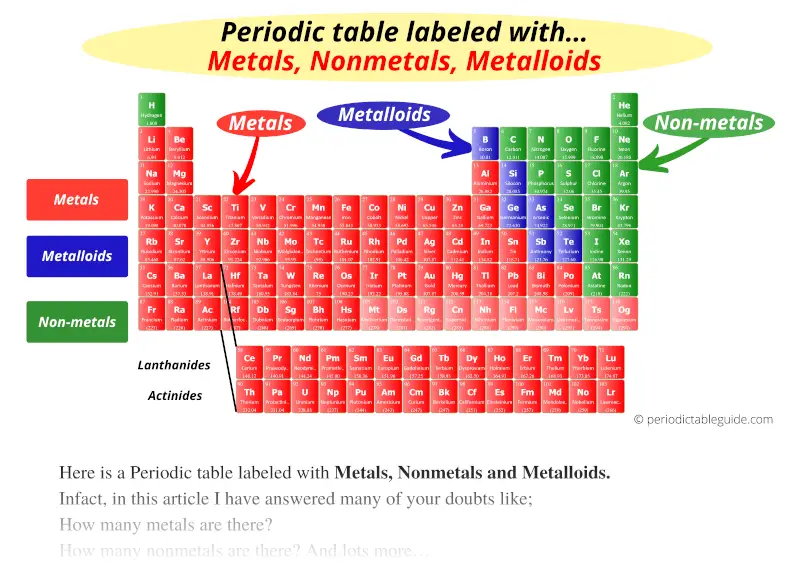
Periodic Table Labeled With Metals Nonmetals And Metalloids
ADescribe three observations seen when lithium reacts with water bDescribe three observations seen when sodium reacts with water.

. The alkali metals react with nonmetals in Groups 15 and 16 Va and VIa of the periodic table. When the alkali metals are cut they initially appear shiny grey but quickly become dull and white as they react with oxygen in the air. Phosphorus form similarly phosphides.
Metals and non-metals can also be distinguished by some chemical properties. In each reaction hydrogen gas is given off and the metal hydroxide is produced. Phosphorus combines with the alkali metals to form phosphides with the general formula M 3 P.
Identify the alkali metal that is most reactive with water. The alkali metals also have low densities. These metals are so reactive that it is necessary to avoid contact with both moisture and oxygen in the air.
The following question is about the reactions of the alkali metals with water. Except for sodium calcium and potassium metals do not combine with hydrogen. Water hydrolyses phosphides to phosphine.
Summary of the Reactions of the First Three Alkali Metals with Water. On reacting with the oxygen they give rise to acidic or neutral oxides. C Describe three observations seen when potassium reacts with water.
Are soft have relatively low melting points. They include lithium sodium and potassium which all react vigorously with water to produce an alkaline solution. They are low enough for the first three lithium sodium and potassium to float on water.
Alkali metals react with atmospheric oxygen and get tarnished of their shining nature. Use the information in the table and the chart to predict the. A term used to describe a material that can be pulled out into a long wire.
Sulfides can be formed by the direct reaction of the alkali metals with elemental sulfur furnishing a variety of sulfides. Describe the reaction of the alkali metals with non-metals. The most common chemical property is the type of oxide that the.
Alkali metals can react with even atmospheric nitrogen to form nitrides. This is known as tarnishing. 62 Recall that alkali metals.
An element in group 2 of the periodic table. Describe the reaction of the alkali metals with non-metals. 3M P M 3 P H 2 O 3MOH PH 3.
On reacting with water they give rise to hydroxides and hydrogen gas. They burn with oxygen to form oxides. The gradual wearing away of a metal due to a chemical reaction.
They combine directly to form salts. Topic 6 - Groups in the periodic table. Of the alkali metals lithium is the least reactive as it is at the.
Non-metals dont react with water. Describe the reaction of the noble gases with metals. Identify the most reactive alkali metal with.
What happens when alkali metals react with nonmetals. An element in Group 1 of the periodic table. 6M N 2 2M 3 N.
Lithium fluorine -- lithium fluoride. Describe the reaction of the alkali metals with non-metals. The Group 1 elements in the periodic table are known as the alkali metals.
Students should be able to describe the reactions of the first three alkali metals with oxygen chlorine and water. Identify a characteristic of halogens. A Li b Na c Cs d K f Rb Hint look at the periodic table and the rows the lower the row meaning the higher number of.
The speed and. Rubidium caesium and francium will react even more vigorously with air and water than the first three alkali metals. But the nature of.
This is due to their lone electron occupying the outermost shell or s-orbital if you. Describe the reaction of the alkali metals with non-metals. You could be asked to describe and explain the reactions of the alkali metals with water.
Alkali metals react directly with all the nonmetals except the noble gases to yield binary ionic compounds containing 1 metal ions. Reactions of alkali metals with water All the alkali metals react vigorously with cold water. Alkali metals often react violently especially so with substances such as water halogens or Group 6 elements.
If i am not mistaken. See the answer See the answer done loading.

The Parts Of The Periodic Table Metals Nonmetals And Metalloids Periodic Table Of The Elements Periodic Table Teaching Chemistry
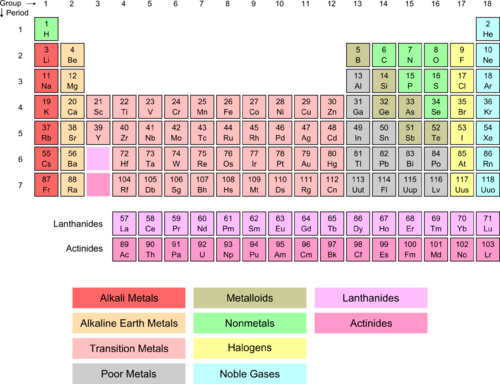
Hydrogen And Alkali Metals Chemistry For Non Majors

Classifying Calm The Chaos Periodic Table Periodic Chart Chemistry
0 Comments